Abstract
Introduction:
Lumbar punctures (LPs) are routinely used to administer intrathecal chemotherapy for children and adults with hematologic malignancies. The platelet threshold at which an LP can be safely performed is a debated topic. A platelet count of ≥50x10 9/L is a widely accepted threshold despite sparse evidence supporting this number (Kaufman et al 2003). Previous studies failed to report any increase in the incidence of post-procedure adverse events in patients with a platelet count below this threshold who undergo LPs (Corroa et al 2018, Chung et al 2020, Öztürk et al 2021). However, these prior studies were limited in their sample size for the number of LPs done in patients with platelets <50 x 10 9/L.
Objectives:
To assess the effects of differing platelet transfusion thresholds (<50 x 10 9/L versus ≥50x10 9/L) in patients undergoing lumbar punctures.
Methods:
We performed a computerized search of Medline, Cochrane and Google Scholar databases through January 2021 for studies evaluating lumbar punctures in adult and pediatric patients. We searched for randomized control trials, non-randomized controlled trials, cohort studies, and retrospective case series. We included any study that involved transfusions of platelet concentrates and was given to prevent bleeding in people of any age with thrombocytopenia that required insertion of a lumbar puncture needle or epidural catheter. We screened 10502 studies and 17 of the studies met criteria for review and were included in the final review. Standard methodological review was completed for each study. Two review authors independently assessed studies for eligibility and extracted data. Results are expressed narratively, and a meta-analysis was performed evaluating traumatic taps with a platelet threshold of <50 x10 9/Lcompared to those over the transfusion threshold of ≥50x10 9/L.
Results:
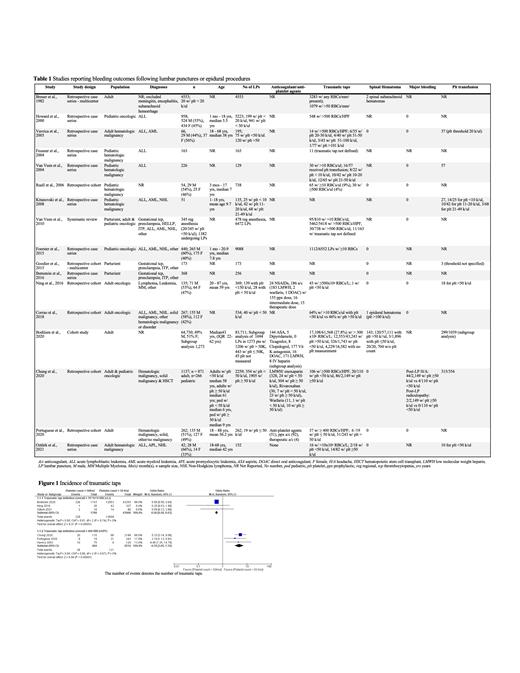
We identified 7 retrospective cohort studies and 10 case series that contained patients who received and did not receive any platelets prior to lumbar puncture procedures (Table 1). Most of the studies (12/17) had a patient population that included an oncologic diagnosis with hematologic malignancies being the most common (Table 1). We summarized the studies in narrative form with relevant outcomes (Table 1).
Spinal hematoma as an outcome was documented in 12/17 studies. There was no difference in spinal hematomas in patients with platelet counts of <50 x10 9/Lcompared to those over the transfusion threshold of ≥50x10 9/L (Table 1).
We performed a meta-analysis in patients with platelet counts of <50 x10 9/Lcompared to those over the transfusion threshold of ≥50x10 9/L for the outcome of traumatic taps. When traumatic taps were defined as greater than 10 x 10 6 RBCs/L, the incidence of traumatic taps favored the patients who had a platelet count ≥50x10 9/L (Figure 1). However, when traumatic taps were defined as >400 RBCs/L the incidence of traumatic taps favored the patients who had a platelet count <50 x10 9/L (Figure 1).
There were no studies that evaluated all-cause mortality within 30 days of the procedure, length of stay, and quality of life outcomes in patients.
Conclusion:
We found no clinical trials to base our recommendations on platelet thresholds prior to a lumbar puncture. We did find multiple studies that should have enough patients to determine if a platelet threshold less than <50 x10 9/L is associated with increased spinal hematomas. There was no increase in spinal hematomas when the platelet count <50 x10 9/L was compared to a cohort with a platelet count ≥50x10 9/L.
The meta-analysis of traumatic taps depended on the definition of traumatic taps and when traumatic taps were defined as greater than 10 x 10 6 RBCs/L, the incidence of traumatic taps favored the patients who had a platelet count ≥50x10 9/L. Whereas, when traumatic taps were defined as >400 RBCs/L the incidence of traumatic taps favored the patients who had a platelet count <50 x10 9/L.
A multicenter prospective study is needed to clarify the incidence of traumatic taps and to verify the data regarding spinal hematomas, other adverse events or quality of life measures in patients.
Laber: Alexion: Membership on an entity's Board of Directors or advisory committees. Visweshwar: Biogen Idec: Membership on an entity's Board of Directors or advisory committees. Jaglal: Novartis: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal